Trends in Oncology
A Step-by-Step Guide to Design a 3D Tumor Model Co-Culture Study
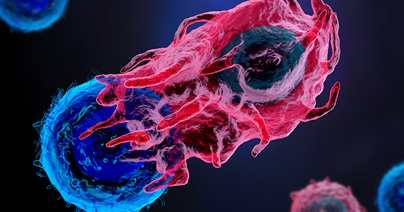
Designing a 3D tumor model co-culture study in oncology research is a meticulous process that demands precision and expertise. However, when executed correctly, these studies provide invaluable insights into tumor-immune microenvironment interactions, potentially leading to groundbreaking therapeutic advancements. This guide outlines essential steps to design a robust 3D tumor model co-culture study, ensuring you capture high-quality data and actionable insights. 1. Select 3D Tumor Models and Validate Target Expression Mechanism of Action: Begin by selecting cancer type, 3D tumor models, and co-culture strategies that are appropriate for the mechanism of action of your therapeutic agent. This involves understanding the biological pathways and processes that your agent targets, ensuring that the 3D tumor models accurately reflect these conditions. Target Expression-Driven Model Selection: Select 3D tumor models based on target expression to best set up your ex-vivo co-culture assay. Leveraging a fully characterized bank, such as Champions’ tumor model bank, is essential for successful model selection. Access to transcriptomic data, for example, allows to rank order the 3D tumor models based on different gene expression profiles, making model selection easy and effective. (Champions’ entire database is accessible for model selection in Lumin) Validation Techniques: Validate target expression using PCR, western blot, or flow cytometry techniques. PCR can help detect and quantify specific nucleic acid sequences, western blotting can confirm protein expression levels, and flow cytometry can analyze the physical and chemical characteristics of cells, ensuring the presence and activity of your targets. This step is crucial for confirming that your targets are being correctly expressed and are functional within your chosen models. Read our blog "Using 3D Ex Vivo Tumor Models for Oncology Research: An Expert Guide" to learn more about the advantages of using tumor organoids in cancer research. 2. Select Multiple Immune Cell Donors to Account for Donor-to-Donor Variability In 3D tumor model co-culture studies, accounting for donor variability is crucial for the robustness of your findings. Selecting immune cell donors from a diverse pool minimizes the risk of skewed results that could arise from individual donor-specific traits. Ensure that immune cells are sourced from multiple donors to account for genetic, epigenetic, and environmental variations. This approach enhances the generalizability and reliability of your study outcomes. Additionally, thorough donor screening and characterization using flow cytometry can further refine your selection process, providing a comprehensive understanding of each donor’s immune profile. Autologous Immune Cells: Autologous immune cells, sourced from the same patient as the 3D tumor model, offer several significant benefits. One of the primary advantages is the enhanced compatibility, as these cells are naturally tailored to interact with the patient’s specific tumor microenvironment. This compatibility can lead to more accurate reflections of in vivo interactions, allowing for better predictive modeling of therapeutic responses. Additionally, using autologous cells can help mitigate the risk of immune rejection, further promoting a more sustained interaction in the 3D tumor model co-culture setting. Despite their advantages, autologous immune cells can present challenges, including donor variability and limited availability. The unique immunological characteristics of each patient can create substantial variability in the efficacy and functionality of the immune cells, leading to inconsistencies in reproducibility. Moreover, some patients may not have accessible immune cells, which can hinder the study's progress and breadth. Allogeneic Immune Cells: On the other hand, allogeneic immune cells, derived from different donors, offer advantages in terms of standardization and availability. These cells can be sourced from healthy donors or patients with known, consistent immune profiles, allowing for high-throughput studies and reproducible results. This standardization can enhance the robustness of the findings, as variations between immune responses can be systematically controlled and compared. However, allogeneic immune cells come with their own set of challenges. The primary concern is the potential for immune rejection, as these cells may not be recognized as 'self' by the 3D tumor model or surrounding microenvironment. This disconnect can influence the dynamics of tumor-immune interactions and may not accurately represent the patient-specific responses, ultimately resulting in less applicable data for therapeutic strategies. Furthermore, the genetic and environmental differences between donors can lead to variability in immune responses, complicating the interpretation of results. Champions supports your ex vivo immuno-oncology program offering both autologous and allogeneic immune cells for 3D tumor model co-culture assays. (In our webinar "Autologous Co-culture Models to Humanized Mouse Models: Navigating IO Models" our experts deep dive into the intricate world of immuno-oncology models to help you identify the best model for your study) 3. Assess Tumor-to-Immune Cell Ratio Achieving the right composition and cell population balance when replicating the tumor microenvironment (TME) ex-vivo is crucial in designing a 3D tumor model co-culture system. One of the strengths of ex-vivo 3D tumor model co-cultures is the ability to customize different parameters to recreate the TME of interest, providing a physiologically relevant platform to predict clinical outcomes. Origin of the Cellular Component and Phenotype: It is very important to source each cell population from the right tissue. When introducing a new cell type within the 3D tumor model co-culture system, it is fundamental to make sure each cellular component is functional and expressing the correct differentiation markers. In some cases, it is necessary to differentiate progenitor cells into the desired cell type before adding them to the 3D tumor model co-culture. In other experimental settings, activating the immune cells to induce specific target expression is crucial to resemble diseased TME. Autologous vs Allogeneic Population Assessment: Verify the necessary stroma and immune cell populations using advanced techniques such as flow cytometry and expression profiling. Flow cytometry allows for the detailed analysis of cell populations, including their size, granularity, and protein expression. Expression profiling further assesses the expression of the correct differentiation markers by stroma cells and the target expression and activation status of the immune cells, ensuring they are primed and ready to attack the tumor cells effectively. Cell Ratios: It's essential to determine the optimal ratio of tumor cells to immune cells to ensure the immune cells can effectively target and eliminate the tumor cells. This involves careful calculation and adjustment based on the specific characteristics of the tumor, TME, and immune cells being used. By carefully balancing cell ratios, type, and status, and thoroughly assessing cell populations, we can control the 3D tumor model co-culture system's ability to evaluate cancer immunotherapies and improve clinical translatability. 4. Identify the Right Assay Controls Controls are pivotal for obtaining reliable results and ensuring the validity of an assay. There are several types of controls to consider: Mechanism-Based Controls: These are crucial for the assay as they should be selected based on the specific mechanism of action of the therapeutic agent being tested. The correct set of controls will allow a better understanding of how the therapeutic agent in preclinical stage of development will behave in the given biological context. In particular, this type of control is essential to interpret mechanistic findings in ex-vivo 3D tumor model co-culture systems. Validation: It is essential to implement these controls consistently throughout the entire assay process. This step ensures that the results are accurate and reproducible, minimizing false positive and negative readouts which is fundamental for drawing meaningful conclusions from the data. In addition, using these controls, researchers can ensure the ex-vivo 3D tumor model co-culture system is responding as expected to a given perturbation and it can provide meaningful results in the specific biological context of interest. By carefully choosing and applying these controls, researchers can significantly improve the reliability and validity of their experimental results. 5. Choose the Best Readout for Tumor Killing Selecting the right readout method is critical for obtaining meaningful data: Imaging: Utilize advanced imaging techniques for visualizing 3D tumor model cell interactions and capturing detailed images that provide insights into cellular behavior and morphology. The high throughput confocal approach provides meaningful images to have a glance into the 3D tumor model co-culture system. Moreover, the image quantification pipeline allows for quantitative data extraction through 3D image reconstruction. Beyond tumor killing, immune cell infiltration into the 3D tumor model is another parameter that can be evaluated through an accurate image analysis. Flow Cytometry: Assess different phenotypes within the diverse cell populations composing the co-culture, viability (which provides a direct tumor-killing measure), and specific target expression using flow cytometry. This technique allows for precise quantification and analysis down to the single cell level, dissecting the heterogeneous populations within the 3D tumor model co-culture system. Luciferase: Employ luciferase-based assays for fast, quantifiable, and direct measurement of cancer cell killing. This endpoint requires the use of luciferase-tagged cells, enabling the measurement of gene expression, cellular activity, and other biological processes through luminescence. (Watch our on-demand webinar "Poster QuickTake: Bioluminescent 3D Tumors with Immune Cell Co-culture for HTS" to learn more about bioluminescence tagged 3D tumor models for co-culture assays) 6. Use Immune Cell Infiltration as an Additional Readout Immune cell infiltration offers deeper insights into the body's response to cancer: Infiltration Analysis: Track immune cell movement into the tumor microenvironment to gauge the effectiveness of your therapeutic agent. This analysis helps in understanding the behavior of immune cells and their interaction with 3D tumor model cells, which is crucial for developing more effective treatments and improving patient outcomes. The 3D organization of the cells in different compartments is important in this kind of analysis to decide which parameter is best to use in evaluating cellular mobility. 7. Assess Immune and Stroma Cell Status Post-Treatment Post-treatment analysis of the 3D tumor model co-culture system helps interpret therapeutic effects by providing detailed insights into cellular and molecular responses. Here are two key techniques: Flow Cytometry: Use this technique to analyze immune and stroma cell phenotypes and statuses. It allows for the identification and quantification of various cell types within a sample, offering a comprehensive overview of changes in the cellular landscape following treatment. Cytokine Analysis: Measure cytokine levels to understand the immune response and stroma cell dynamics post-treatment. By quantifying specific cytokines, researchers can infer the activation and regulation of different immune pathways, shedding light on the effectiveness and impact of the therapeutic intervention. Designing a 3D tumor model co-culture study is a complex yet rewarding endeavor that can yield significant insights into tumor biology and treatment efficacy. By following these steps, you can ensure your study is thorough, accurate, and highly informative. Ready to take your 3D tumor model co-culture studies to the next level? Partner with Champions Oncology for unrivaled expertise and cutting-edge resources to propel your research forward. We tailor our services to meet your specific research needs. Contact us today to learn more!
How to use metadata as your model selection GPS
In the complex landscape of oncology research and drug development, precision is paramount. The ability to select appropriate models can significantly impact the success and validity of your research. This is where metadata steps in as your reliable guide for model selection. Understanding Metadata in Research Metadata, essentially data about data, plays a crucial role in organizing, describing, and finding datasets. In the context of oncology research, metadata encompasses all the details about experiments, datasets, and results. This includes information on sample types, experimental conditions, data collection methods, and software used. By providing a structured summary of data, metadata helps streamline the process of data retrieval and utilization, for model selection and complex data analysis. Metadata serves as a roadmap, guiding researchers to the datasets they need, while also providing context and ensuring that data is used appropriately. It’s an essential tool for maintaining consistency and transparency in research. The better structured and more detailed the metadata, the easier it is to manage and interpret large datasets, which are common in oncology. In oncology research, metadata might include details such as the type of tissue samples collected, the sequencing methods used, and the specific conditions under which experiments were conducted. This ensures that datasets remain comprehensible and reusable, even years after the initial research was completed. The Importance of Accurate Metadata in Model Selection Accurate metadata is the backbone of effective model selection. It provides the necessary context to understand datasets fully, ensuring that models are selected based on relevant and precise information. This is particularly important in oncology research, where the accuracy of findings can have significant implications for patient outcomes. Champions Oncology’s entire database can be explored accessing Lumin, our proprietary integrated software solution that harnesses the power of precisely curated oncology data, computational science, and bioinformatics to help you make data-driven decisions in model selection and data interpretation. Within Lumin you can: visualize proprietary genomic, proteomic, and pharmacological profiles from Champions’ patient-derived preclinical models; generate analyses on unique multi-omic datasets assembled from over 12,000 patients, including thousands of clinical treatment responses not available in public databases; interrogate experimental data and accelerate your oncology research programs. Enhancing Model Selection With accurate metadata, researchers can filter through vast amounts of data to find the most relevant datasets for their specific needs. For instance, if you're looking for data on a particular cancer type and drug target, well curated metadata can help you quickly identify and access the relevant datasets, saving time and resources. Scientists planning to study the efficacy of a novel targeted therapy developed to treat tumors of a specific type that are resistant to the standard of care for that tumor type could use metadata to select the best set of PDX models to use in their study. Within Lumin, they could filter our bank of tumor models by tumor type first, and then, by drug response profile. In the example below, we selected all NSCLC PDX models derived from patients who were pre-treated with osimertinib and that did not respond to osimertinib treatment in vivo. This set of models would well represent the target patient population for our drug. Additionally, scientists could further select models based on gene mutation and RNA and/or protein expression levels of their agent’s target. A deeper look into the tumor model metadata, leveraging data analysis and visualization tools, can help fine-tuning model selection further improving the quality of the study results. Deep characterization and extensive clinical annotation of patient-derived tumor models, allows for a more precise model selection, which translates into a well-designed mouse clinical trial that closely reproduces the clinical setting and patient target population, and ultimately leads to more accurate results and a faster track to clinical experimentation. Best Practices in Structuring Metadata To harness the full potential of metadata, it's essential to follow best practices in its structuring and management. Here are some key guidelines for oncology researchers. Standardized Formats Using standardized formats for metadata ensures consistency and compatibility across different datasets and platforms. This makes it easier to integrate and compare data from various sources, enhancing the overall quality and reliability of your research. Detailed Descriptions Detailed descriptions of the datasets, including information on the origin of the data, the methods used for collection and analysis, and any relevant environmental or experimental conditions are very important. The more detailed your metadata, the more useful it will be for model selection and to bridge with future research. Regular Updates Keeping metadata up-to-date to reflect any changes or new findings is paramount. Regular updates ensure that data remains relevant and accurate, supporting the ongoing use and application of any research findings. Future Trends in Metadata for Research and Biotech The landscape of metadata is continually evolving, driven by advances in technology and increasing demands for data accuracy and transparency. Here are some future trends that are likely to shape the use of metadata in oncology research. AI and Machine Learning Artificial intelligence (AI) and machine learning are set to revolutionize the way we manage and utilize metadata. These technologies can automate the process of metadata generation and analysis, making it faster and more efficient. For example, AI algorithms can analyze large datasets to identify patterns and correlations that might be missed by human researchers. Integration with Big Data As the volume of data in oncology research continues to grow, there will be an increasing need for robust metadata systems that can handle big data. Integrating metadata with big data platforms will enhance the ability to manage, analyze, and interpret vast amounts of information, leading to more accurate and comprehensive research outcomes. Metadata is an invaluable tool for oncology researchers, providing the context and structure needed to manage complex datasets effectively. By following best practices in model selection, you can enhance the accuracy and efficiency of your research, leading to better outcomes and discoveries. The incorporation of metadata into your research processes not only supports better model selection and data integrity but also fosters collaboration and innovation. Ready to advance your oncology pipeline with faster model selection and AI-powered data analytics & visualizations? Sign up for our free trial of Lumin and discover how we can help you streamline your model selection and accelerate your discoveries.
5 Essential Steps for a Successful Immuno-Oncology In Vivo Study
Conducting an Immuno-Oncology in vivo study is a complex process that requires meticulous planning and execution. For oncology researchers and biotech professionals, ensuring the success of these studies is crucial for advancing cancer treatments. Here are the five essential steps to guide you through a successful immuno-oncology in vivo study. 1. Select the Best Tumor Models One of the first critical steps is choosing the appropriate tumor models. This involves: Molecular Data Analysis: Leverage molecular data to select tumor models that express your drug target and most closely represent the target patient population. Ex Vivo Screening: Utilize ex vivo screening techniques to evaluate the effectiveness of your drug in various tumor models and select models with the best response profile. By selecting the most relevant tumor models for your immuno-oncology in vivo study, whether Patient-Derived Xenografts (PDXs) or cell lines, you can ensure that your immuno-oncology in vivo studies will provide more accurate and translatable results. Verified target expression via proteomics and genomics data, and selection of pretreated or naïve models are two extremely important elements of model selection. 2. Choose the Right Mouse Model The choice of mouse model can significantly impact the outcomes of your immuno-oncology in vivo study. Below we provide some directions on how to navigate the different available mouse model options. Murine immune system: Syngeneic Mouse Models: These are generally used when the candidate immuno-oncology drug is able to interact with the murine version of the human target. These models are generated by inoculating murine cell lines into the mice, providing a controlled environment to study immune response. Knock-in Humanized Mouse Models: These models are used when the candidate immuno-oncology drug only interacts with the human target molecule. These models are generated by inserting human genes into the mouse genome, allowing the candidate drug to target the murine immune response. Humanized immune system: Adoptive transfer humanized models: This group of humanized mice allows for the engraftment of mature immune cells such as PBMCs and NK cells. The experimental strategy consists of engrafting human immune cells isolated from peripheral blood in immune-compromised mice to mimic some aspect of the human immune response. CD34 HSC humanized model: This approach provides a platform closer to the human immune system by engrafting CD34 hematopoietic stem cells (HSC) from the human cord blood. The presence of the main populations of the human immune system, including both innate and adaptive immune players, ensures an ideal platform to test a variety of immune-targeting drugs. Selecting the right mouse model for your immuno-oncology in vivo study is crucial for understanding how your treatment will interact with the immune system. (Read our blog "Choosing the RIGHT Model - Syngeneic versus Humanized Mouse Models") 3. Evaluate Drug Toxicity Before proceeding with efficacy studies, it's essential to evaluate the safety of your drug in the chosen mouse model system: Acute Toxicity Testing: Assess the immediate effects of your drug on the mouse models to identify any potential adverse reactions. Usually, a single high dose of the agent is administered to the animals, and toxicity is evaluated within 24 hours from drug administration. Chronic Toxicity Testing: Examine the long-term effects to ensure the drug is safe for prolonged use. In this case, animals are exposed to repeated lower doses of the candidate agent to assess toxicity over a longer period of time. Ensuring the drug's safety through comprehensive toxicity testing is vital for the integrity of your immuno-oncology in vivo study. 4. Evaluate Tumor Killing Next, conduct tumor-killing studies to evaluate the efficacy of your treatment: In Vivo Efficacy Testing: Measure the tumor size reduction and observe the mouse models' overall health. Comparison Studies: Compare your drug’s performance with existing treatments to establish its effectiveness and advantage over standard of care therapies. These studies will provide concrete evidence of your immuno-oncology drug's potential to kill cancer cells, a fundamental step before moving to the clinical research stage. 5. Analyze the Mechanism of Drug Action Understanding how your drug works at a molecular level is the final step to creating a successful immuno-oncology in vivo study. Flow Cytometry: Use flow cytometry to analyze the types and states of cells affected by your treatment. This analysis will reveal the effects of your candidate drug on the number, proliferation, and activation of the different immune cells. (Read our blog "Using Flow Cytometry as an In Vivo Study Endpoint") RNA Sequencing (RNA-seq): Perform RNA-seq to examine gene expression changes induced by your drug. Complementary to flow cytometry, RNA-seq, and DRUG-seq can expose the on-target and off-target effects of your candidate drug, possibly revealing new therapeutic and synergistic opportunities. (Read our blog "Exploring DRUG-seq: Revolutionizing RNA-seq in Oncology Research") These analyses will give you insights into the precise mechanisms through which your immuno-oncology drug operates, paving the way for further development and optimization. By following these five essential steps, you can enhance the accuracy, efficacy, and safety of your immuno-oncology in vivo study. At Champions Oncology, we are committed to supporting researchers with cutting-edge tools and expertise. Ready to take your research to the next level? Reach out to our team of experts and see how we can help you achieve groundbreaking results.
Development of Innovative Therapies Utilizing Preclinical CLL Models
Did you know that Chronic Lymphocytic Leukemia (CLL) is a relentless adversary in the realm of hematologic malignancies? Characterized by the excessive accumulation of abnormal B lymphocytes, CLL presents a unique set of challenges for oncologists, hematologists, and cancer researchers. Despite advancements in targeted therapies and immunotherapies, achieving sustained remission remains elusive for many patients. In this blog, we will explore the critical role preclinical CLL models play in the development of novel therapies. From understanding the disease's biology to facilitating the development of breakthrough treatments, these models are indispensable tools in our fight against CLL. The Role of Preclinical CLL Models in Cancer Research Preclinical CLL models are the unsung heroes of CLL research, providing a crucial bridge between laboratory discoveries and clinical applications. These models enable researchers to study the complex biology of CLL in a controlled environment, offering insights that are impossible to glean from human studies alone. By replicating the disease in animals or in culture with preclinical CLL models, scientists can test the efficacy and safety of potential therapies before they reach clinical trials. This accelerates the drug development process and enhances our understanding of disease mechanisms. In essence, preclinical CLL models are the bedrock upon which modern CLL cancer therapies are built. Overview of Current Preclinical CLL Models The landscape of preclinical CLL models is diverse and continually evolving. Each model offers unique advantages and limitations, making choosing the right one for specific research objectives essential. Cell line-derived Xenograft (CDX) Models Xenograft models involve transplanting human CLL cells into immunodeficient mice. These preclinical CLL models allow for the study of human-specific disease characteristics and the evaluation of human-targeted therapies. However, the lack of a fully functional immune system in these mice limits the study of immune-based treatments. Genetically Engineered Mouse Models (GEMMs) GEMMs are designed to mimic the genetic aberrations found in human CLL. These preclinical CLL models provide a more accurate representation of the disease's progression and response to therapies. They are particularly valuable for studying the genetic and epigenetic factors driving CLL. Patient-Derived Xenografts (PDXs) PDXs involve implanting primary CLL cells from patients into mice and serially passaging to obtain stable in vivo models[1]. These preclinical CLL models retain genetic features of the original tumor, making them predictive of clinical outcomes, although the lack of a proficient immune system needs to be considered. Using Preclinical CLL Models in Oncology Research The latest therapies approved for CLL are a testament to the power of preclinical CLL models in developing revolutionary therapies. Pirtobrutinib (approved by the FDA at the end of 2023[2]) and lisocabtagene maraleucel (approved by the FDA for use in CLL in 2024[3]) were meticulously tested in preclinical CLL models before their clinical debut, ensuring their safety and efficacy in targeting CLL cells. Pirtobrutinib is a highly selective, noncovalent Bruton tyrosine kinase inhibitor (BTKi). Preclinical testing of pirtobrutinib was conducted in several preclinical CLL models. CLL cell lines were used to assess target engagement, potency, cellular phosphorylation, and other cellular activity of the inhibitor[4, 5, 6]. Further studies in primary CLL cells and xenograft models confirmed pirtobrutinib's ability to kill CLL cells and reduce tumor burden[4, 6]. Lisocabtagene maraleucel is a CD19-targeted CAR-T cell therapy. Preclinical studies of lisocabtagene maraleucel involved in vitro and xenograft models to evaluate its ability to target and eliminate CLL cells[7, 8]. These preclinical CLL models provided crucial data on the therapy's potency, specificity, and potential side effects. The success of these preclinical trials paved the way for pirtobrutinib and lisocabtagene maraleucel approval and use in clinical settings. These therapies are now transforming the treatment landscape for CLL and other hematologic malignancies. The Impact and Future of Preclinical CLL Models in Developing Novel Therapies The impact of preclinical CLL models on the development of new therapies cannot be overstated. They have accelerated the discovery of novel treatments, reduced the risk of adverse effects, and improved patient outcomes. However, the field is far from static. Advancements in Model Precision The future of preclinical CLL models lies in their ability to reproduce clinical characteristics and support personalized medicine. The use of primary patient-derived CLL models by direct injection of patients’ cells in mice without additional passages in the animals allows better preservation of tumor heterogeneity and patient population diversity[9], Patient-derived CLL models can be used in a preclinical trial format as well as for the testing of therapies on individual patient tumors, enabling tailored treatment strategies. The development of such models in humanized mice, which possess a reconstituted human immune system, will further improve the robustness of these models as patients’ surrogates by enabling a deeper understanding of how therapies interact with the human immune system, leading to more effective treatments. Integration of Computational Models Integrating computational models with preclinical studies is another promising avenue. By simulating disease progression and treatment responses in silico, researchers can optimize experimental designs and predict outcomes more accurately. This synergy between computational and experimental approaches is poised to accelerate the development of next-generation CLL therapies. The Importance of Continued Research and Innovation Preclinical CLL models are the linchpin of CLL therapy development, providing invaluable insights and accelerating the transition from bench to bedside. As we continue to refine these models and integrate new technologies, the future of CLL treatment looks increasingly promising. Champions Oncology's bank of preclinical CLL models includes primary models derived from pretreated and naive patients. With deep multi-omic and multimodal characterization and comprehensive clinical annotations, we strive to make our preclinical CLL models the best tool to accelerate your drug pipeline through reliable data and extensive expertise in the hematologic malignancies field. Contact us to speak with one of our experts.
Using 3D Ex Vivo Tumor Models for Oncology Research: An Expert Guide
Did you know that 3D organ-like tumor models are biomimetic and yield superior results in drug screening? These models more accurately mimic cell-cell signaling and physiological conditions, providing a superior representation of human tumors outside the body. This blog is dedicated to answering frequently asked questions about Champion's advanced TumorGraft3D platform and assisting scientists in choosing the right platform for their drug screening endeavors. Champions Oncology offers the multiclonal TumorGraft3D drug screening platform, a biologically relevant platform with 3D organoid models derived from our superior, well-characterized patient-derived xenografts (PDXs) for ex vivo drug testing. 1) What is TumorGraft3D and what differentiates them from conventional 3D models? TumorGraft3D models are self-organizing three-dimensional PDX-derived cell clusters that mimic parental human tumor's morphological and molecular phenotype, thereby rendering themselves clinically relevant models for drug discovery. 2) What types of TumorGraft3D models are currently available? Our TumorGraft3D biobank is constantly expanding and currently includes over 150 off-the-shelf models across 16 different tumor types for ex vivo studies. However, our entire PDX bank is available for TumorGraft3D generation, only requiring a short pre-study development phase before becoming available for clients’ studies. 3) What are the advantages of using TumorGraft3D models compared to cell lines or in vivo PDX studies? TumorGraft3D models are generated from our deeply characterized and clinically annotated patient-derived xenograft models and maintain the parent model’s characteristics. They represent the heterogeneity of the patient population and have drug response profiles comparable to the parent PDX model. This makes them a great resource for ex vivo drug screening, with better clinical correlation than cell lines, and have a faster turnaround time and higher throughput than in vivo studies. 4) Are TumorGraft3D models a close representation of patients’ tumors? How do you ensure that patients' characteristics are not lost? TumorGraft3D models are derived from our PDX models without intermediate processing steps and are maintained at low passages to avoid genetic drift and preserve the parent PDX model characteristics. Moreover, IHC, NGS, and drug response analysis are conducted to characterize the TumorGraft3D model and verify that this maintains the parent PDX model’s molecular and pharmacological profile. 5) Why do you use a matrix-free assay? What are the advantages and disadvantages of it? Traditionally, 3D tissue models have been cultured using an extracellular matrix-dependent approach, where the supportive extracellular matrix is derived from natural or synthetic sources. Matrices can pose several challenges such as sourcing of materials, interactions with test agents, and interference in imaging-relevant readouts. Matrix-free approaches allow the end user to observe the self-organization of 3D tissue models without using an exogenous matrix, circumventing all the above challenges. A matrix-free environment allows homogeneous distribution of therapeutic agents and easy access to tumor and immune cells. Additionally, the absence of confounding factors simplifies the assay readout and reduces variability. The use of an exogenous matrix can mimic the physical characteristics of the TME but can also influence tumor cell behavior and it interferes with flow cytometry readouts. 6) How can I use TumorGraft3D to study the TME and agents targeting/engaging the TME? TumorGraft3D models are a well-defined versatile platform that lends itself to various co-culture options and readouts that can generate a complete picture of the TME response to test agents. At Champions, we offer co-culture assays with autologous and allogeneic NK cells, PBMCs, TILs, and other immune cells, allowing for testing of several classes of therapeutic agents such as T cell and NK cell engagers, BiTEs, ADCC drugs, immune checkpoint inhibitors, small molecules, gene therapy, cell therapy, and combinatorial therapy. 7) What are the applications of TumorGraft3D? How can I use this platform to advance my oncology research program? TumorGraft3D models' versatility and clinical relevance, along with their molecular characterization, make them the ideal ex vivo models to measure agent efficacy and on-target effect, unravel the complexities of tumor biology and the crucial interactions within the tumor microenvironment, and evaluate synergistic combination therapies to de-risk in vivo studies through data-driven decisions. 8) How does Champions Oncology ensure the quality of your 3D tumor models? At Champions, we strive to provide the highest quality services. Our TumorGraft3D models are carefully developed following cutting-edge guidelines for organoid development and culture. Our models are: • maintained at low passages to retain molecular and phenotypic characteristics, • qualified for assays using predefined SOC compounds, • verified for true 3D structure formation and viability throughout the length of the assay. 9) What is unique about the TumorGraft3D platform? Specially tailored for integration with various immune cell types, TumorGraft3D models incorporate the unparalleled advantage of Champions’ proprietary autologous systems. Our proprietary autologous co-culture systems proficiently examine the potency of therapeutic agents alongside the intricate interactions between a patient's tumor and their own immune system. This advancement eliminates the inconsistencies commonly seen with the use of allogeneic donors, directing the focus onto patient-specific responses. Our clinically relevant models, coupled with advanced high-content imaging and flow cytometry immunophenotyping make our platform a rich source of insights. With unprecedented precision, scientists can now: • monitor the influence of test agents on the interaction between tumors and their microenvironment, • decrypt their mechanisms of action, • meticulously track the infiltration of immune cells, • and craft potent combination therapies tailored for IO applications.

Navigating Clinical Specialty Testing: Key Insights into Regulatory Compliance
Clinical specialty testing laboratories, like Champions Oncology, are expected to adhere to stringent standards to ensure accuracy and reliability of test results which can have life-altering implications for patients. Regulatory compliance is not a mere bureaucratic hoop but a foundational element that guarantees the integrity of laboratory operations. Navigating through the complex landscape of clinical specialty testing and its regulatory environment is crucial for the success of each clinical trial. Regulatory compliance within each clinical trial is vital to ensure data validity and also ensures each laboratory’s commitment to patients’ safety. In this blog post, we’ll explore the intricacies of adhering to Good Clinical Laboratory Practice (GCLP), Clinical Laboratory Improvement Amendments (CLIA), and College of American Pathologists (CAP) standards, compare regulatory frameworks in the United States (US) versus the European Union (EU) and underscore why meticulous regulatory compliance is a non-negotiable for each clinical trial. Clinical specialty testing laboratories, like Champions Oncology, are expected to adhere to stringent standards to ensure accuracy and reliability of test results which can have life-altering implications for patients. Regulatory compliance is not a mere bureaucratic hoop but a foundational element that guarantees the integrity of laboratory operations. Good Clinical Laboratory Practice (GCLP) GCLP is a quality system that ensures laboratories conducting clinical trial testing provide data of consistent quality. It bridges the gap between the guidelines provided by Good Laboratory Practice (GLP) and Good Clinical Practice (GCP), focusing on pre-analytical, analytical, and post-analytical processes. Clinical Laboratory Improvement Amendments (CLIA) In the United States, CLIA regulations pertain to laboratory testing and require labs to be certified by the federal government. They establish standards for test performance, personnel qualifications, quality control, and proficiency testing for each specific assay performed at the specialty testing laboratory. College of American Pathologists (CAP) The CAP accreditation is an internationally recognized program that provides a framework for clinical labs to achieve excellence in patient care and ensure compliance with statutory and regulatory requirements. CAP takes a peer-reviewed approach to help maintain the highest standard of care. While there may be considerable overlap in what these regulations and standards aim to achieve, there are nuanced differences in their requirements and scopes. GCLP is broader and more flexible in its application, potentially accommodating international guidelines. CLIA is prescriptive and specific to the United States, focusing significantly on the analytical phase of testing. CAP, albeit a US-based program, aligns with many international standards and offers a comprehensive accreditation process that envelopes all aspects of lab operations. Comparatively, the European Union (EU) takes a different approach to laboratory oversight. The EU mandates that each company ensures the quality and safety of its laboratories, but it does not impose a uniform set of standards. Instead of an EU-wide equivalent to CLIA, countries may have their own regulatory frameworks or adhere to international standards like those of the International Organization for Standardization (ISO). Regulatory standards are the pillars that support the validity of clinical trial data. They are key to ensuring that the specialty tests upon which clinical decisions are based are reliable and reproducible. Compliance ensures patient safety, the validity of data submitted to regulatory authorities, and ultimately the success of a clinical trial. Failures in compliance can lead to serious legal consequences and ethical breaches, undermining public trust. Every clinical scientist must understand that regulatory compliance is not simply about following rules; it's about upholding the scientific rigor and ethical duty inherent in clinical research. Each standard, whether it be GCLP, CLIA, or CAP, serves as a QA/QC mechanism to this end. By mastering these regulatory frameworks and recognizing their importance in every aspect of a clinical trial, we safeguard the integrity of clinical research, protect patient welfare, and contribute to the greater good of advancing scientific clinical research.
Accelerating Innovation & Drug Development with Pre-treated PDX Models
The landscape of oncology research and the quest for better treatments have intensified with the advent of precision medicine introducing more targeted agents with higher efficacy and lower toxicity compared to traditional chemotherapy. In personalized medicine, where tailored treatments are revolutionizing patient care, understanding drug resistance mechanisms is key to developing more effective therapies. This blog takes a deep dive into pre-treated patient-derived xenograft (PDX) models, explaining what they are and how they are employed in preclinical studies to combat resistance to standard of care drugs, ultimately leading to better patient outcomes. Refining Precision: PDX Models in a Nutshell PDX models involve the transplantation of human cancer tissue into immunodeficient mice for in vivo growth and expansion. The resultant 'model' is a living tumor system that retains molecular and cellular characteristics closely resembling those of the original patient tumor. Compared to traditional cancer cell line models, PDX models are superior avatars as they capture the heterogeneity and microenvironmental cues found in human tumors. This fidelity allows for a more accurate predictive platform to assess drug responses in preclinical oncology research. Unveiling Resistance: The Power of Pre-treated PDX Models In the quest to outwit cancer, the central challenge has always been predicting and overcoming therapeutic resistance, which often arises after initial treatment with standard of care drugs. Pre-treated PDX models are established from tumors from patients that have previously been exposed to treatments, thus embodying resistant tumor phenotypes that can mimic clinical presentations. The use of PDX models after exposure to specific lines of chemotherapy, hormone therapy, or targeted drug regimens reveals a wealth of insights into how these tumors adapt, evolve, and evade the therapeutic effects. This critical approach allows researchers to identify the genetic and molecular determinants of resistance and devise strategies to combat them. Pre-treated PDX Models at Champions Oncology Champions’ highly characterized biobank includes over 1,000 tumor models across 60+ tumor types derived from advanced stage primary and metastatic patient tumors pre-treated with a plethora of therapies including the latest generations of targeted antibodies and small molecules, immune checkpoint inhibitors, ADCs, bispecific antibodies, and even experimental therapies currently undergoing clinical trial. Our pre-treated models can be easily identified in Lumin, where you can select models of interest to you based on clinical and molecular characteristics, and draft a PDX cohort for your next study. With extensive longitudinal clinical annotations and industry-leading multi-omic and multi-modal characterization, Champions' pre-treated tumor models allow for testing therapeutic agents in systems that closely mirror the clinical trial patient population significantly derisking the drug development process. Transforming Resistance to Resilience in Research Pre-treated PDX models have already catalyzed groundbreaking advancements in oncological research. For instance, studies have utilized these models to identify secondary oncogenic events that drive drug resistance and to develop combinatorial therapies that prevent or even reverse drug insensitivity. One of these studies is highlighted in our case study "Using Champions’ patient-derived xenograft (PDX) models for preclinical validation of HER2-specific small molecule inhibitors" where tucatinib is used as a single agent or in combination with trastuzumab in a selection of HER2-amplified PDX models, both pre-treated and naïve, showing tumor inhibition in trastuzumab-resistant models. The data derived from pre-treated PDX models can be integral in informing personalized treatment strategies for cancer patients. The Future of Research and Care in Oncology The implications of utilizing pre-treated PDX models are not just confined to the benches of research labs. These models have the potential to significantly impact the course of clinical trials, where they can serve as powerful tools to validate therapeutic responses and study mechanisms of drug failure in a more controlled environment. Investigating drug resistance in PDX models allows for a more systematic and accelerated approach to drug development. New compounds can be tested on pre-treated PDX models to evaluate their potential benefits, potential interactions with existing therapies, and their ability to overcome resistance, ensuring a fast track to clinical trial. By providing a more accurate representation of human tumors and treatment responses, pre-treated PDX models bridge the gap between bench-side discoveries and clinical applications. This synergy is crucial in ensuring that the most promising treatments reach patients in a timely and effective manner.

Deciphering CNV: Utilizing Gene Copy Number Variation Data in Lumin
Gene copy number in a tumor cell is a significant indicator of the implication of a given gene in several oncogenic processes such as uncontrolled proliferation, elusion of programmed cell death, and resistance to treatments. At Champions Oncology, gene copy number analysis is performed by using the EXCAVATOR2[1] tool on whole exome sequencing (WES) data generated to characterize our patient-derived xenograft (PDX) models. This tool allows for classifying each segmented region into five qualitative genomic states (two-copy deletion, one-copy deletion, normal, one-copy duplication, and multiple-copy amplification) and quantifying the number of chromosomal copies. All our model characterization data can be explored in Lumin, a unique solution integrating Champions’ tumor model multi-omic data and public datasets in one accessible platform for model selection and data interpretation. In Lumin, gene copy number analysis results are presented in the format shown in the example below: Here we answer the most common questions about CNV data reporting to help you navigate WES data in our Lumin platform as well as interpret your own study data. Q: What does Log2R mean and why sometimes is it marked as NA? A: The Log2R (Log2 ratio) value represents the number of copies relative to the normal reference sample (NA12878). The EXCAVATOR2 algorithm used to calculate CNV uses a median normalization approach, with the log-transformed ratio (Log2R) being calculated from the window mean read count (WMRC) values of the test sample compared to the normal reference. When Log2R value is marked as NA, no significant copy number alteration was detected. Q: What are the Call values and how are they defined? A: The Call value is calculated using the FastCall algorithm[2] and classifies each segmented region as one of five possible states: 2 copy deletion= -2; one copy deletion= -1; normal= 0; one copy duplication= 1; and multiple copy amplification= 2. Q: Are the copy number values the absolute or the relative copy numbers detected? A: Copy number values represent the absolute copy number detected, which is derived from the Copy Number Fraction and is rounded to the nearest integer. Q: Do “Amp” and "Del" always mean that there is a gain or loss in copy number? Or is this only the case if the “Alteration” column says “Gain” or “HomoDel”/ “Hetloss”? A: The "amp_del" column definitions are derived from the Call values, whereas the "Alteration" column classifies the alteration based on the copy number detected. In some instances, there may be discordance between the copy number and Call (as shown for FAM231C gene in the example table above), as the two values are derived by approximation of continuous values. In this specific example, the conflict between the two annotations is indicative of the tumor gene copy number being between 1 and 2, suggesting the presence of both cells with 1 and cells with 2 copies of that genomic region. For additional investigation, we recommend looking at the continuous values in the raw data. Q: What columns should I consider when I want to search for a model with an amplification/deletion for a certain gene? A: We would first recommend using the "CopyNumber" column to identify models with an amplification or deletion of a specific gene. Once you have filtered the models based on this, you can then use the “Alteration” column to verify whether Call, copy number, and the “amp_del” column values are concordant.
The Evolution of Treatment in TNBC: A Closer Look at Pembrolizumab
In the realm of oncology, few words instill as much uncertainty and trepidation as "triple negative breast cancer" (TNBC). With its resistance to many standard forms of therapy, TNBC demands a new generation of treatment innovation. Enter pembrolizumab—an immunotherapy designed to engage the body’s immune system in the fight against cancer. Its recent breakthroughs in clinical trials have charted a promising new course in the treatment of this aggressive form of breast cancer. The TNBC challenge Conventionally, breast cancer treatment plans are designed around the presence or absence of three receptors: estrogen, progesterone, and HER2/neu. TNBC, characterized by the absence of these receptors, compels a more bespoke approach, given the limitations it imposes on targeted treatments available for other forms of breast cancer. The lack of defined therapeutic targets has historically left TNBC patients with fewer options and a greater risk of disease progression and poor prognosis.[1] The arrival of pembrolizumab: a precision tool in the TNBC arsenal Pembrolizumab operates on a fundamentally different principle than traditional treatments. It is an immune checkpoint inhibitor that effectively releases the brakes on the immune system, allowing it to identify and combat cancer cells in a manner that is highly specific to a patient's tumor profile. This tailored approach has been nothing short of revolutionary in cancers with high mutational loads, such as TNBC, where the potential for an immune system response is significant.[2] Groundbreaking trials: pembrolizumab's journey to TNBC approval KEYNOTE-355 The KEYNOTE-355 study burst into the scientific limelight with its findings on the efficacy and safety of pembrolizumab in combination with chemotherapy in the treatment of locally recurrent inoperable or metastatic TNBC. The trial's incorporation of a diverse patient population, coupled with the comprehensive analysis of the drug's performance set a new precedent for the depth and breadth of oncology research.[3] This landmark study, along with other trials such as KEYNOTE-012 and KEYNOTE-086, has served as evidence in the FDA's approval of pembrolizumab in combination with chemotherapy for the treatment of advanced TNBC expressing high levels of PD-L1. These studies have demonstrated not only the drug's ability to improve patient outcomes but also its potential to revolutionize the treatment landscape for this challenging form of breast cancer.[4] The KEYNOTE-355 study has been a beacon of hope for those combating triple-negative breast cancer, illuminating the path forward with its groundbreaking results. Central to its findings is the remarkable improvement in overall survival (OS) rates for patients treated with pembrolizumab in conjunction with chemotherapy. Specifically, the study delineates a median OS of 23 months for these patients, significantly surpassing the 16.1 months median observed in those receiving chemotherapy alone. This stark contrast not only emphasizes pembrolizumab's efficacy but also marks a tangible advancement in extending the lives of individuals facing this formidable adversary.[4,5] KEYNOTE-522 Similarly, the KEYNOTE-522 trial, which investigated pembrolizumab in the neoadjuvant setting, demonstrated a significant pathologic complete response (pCR) rate. This paradigm-shifting evidence supported the FDA's approval of pembrolizumab for high-risk early-stage TNBC, leading to a pivotal shift in the narrative around treating this formidable adversary.[6] The results from KEYNOTE-522 have been nothing short of revolutionary, illustrating a marked improvement in both pCR (7.5% higher than in the control arm) and event-free survival (EFS). Patients on the pembrolizumab arm experienced EFS benefit regardless of tumor PD-L1 status.[6,7] What makes these results particularly compelling is the implication for long-term survival outcomes. Early indications suggest that the increase in pathologic complete response rates correlates with longer overall survival, offering new hope that pembrolizumab could extend lives in a population historically challenged by high relapse and mortality rates. These outcomes underscore the importance of pembrolizumab in the TNBC treatment paradigm, highlighting its potential to significantly alter the prognostic outlook for patients facing this aggressive form of breast cancer.[6,7] The future of the TNBC treatment landscape The integration of pembrolizumab into TNBC treatment protocols is a landmark event that underscores the ongoing refinement of personalized oncology care. While the successes in clinical trials are incredibly encouraging, the implementation of these new standards into broader clinical practice requires deliberate consideration of patient selection, administration protocols, and the management of potential immune-related adverse events.[8] Researchers and clinicians are now tasked with harnessing the full potential of this breakthrough treatment as well as identifying novel strategies to extend patient survival and improve quality of life. The future of TNBC treatment lies in leveraging emerging technologies and collaborative frameworks to uncover even more effective therapeutic solutions.
Immune Checkpoint Blockade Strategies in Renal Cell Carcinoma
Renal cell carcinoma (RCC) is a common cancer of the genitourinary tract that has very poor survival outcomes if metastatic. RCC is now understood to be composed of several different types of cancer with different genetic features and varied clinical responses. Histological diagnosis has been the primary method to diagnose RCC and has been used to define three major RCC subtypes, including the most common subtype, clear cell renal cell carcinoma (ccRCC), papillary renal cell carcinoma (PRCC; further divided into two subtypes), and chromophobe renal cell carcinoma (ChRCC)[1]. More recent comparative genomic and phenotypic analysis has identified mutations and epigenetic modifications associated with different histological subtypes[2]. Across all subtypes, increased DNA hypermethylation and gene alterations in CDKN2A were associated with a poor prognosis as was an increased Th2 immune gene signature. For ccRCC, increased levels of mRNA transcripts associated with ribose metabolism and the immune response were associated with poor survival. ccRCC is also defined by the early loss of chromosome 3p, which in turn causes a loss of heterozygosity for the VHL, PBRM1, SETD2, and BAP1 tumor suppressor genes and subsequent mutation of these genes that leads to tumorigenesis[3]. There is also a subset of ChRCC with a unique metabolic expression pattern that is associated with extremely poor survival[2]. PRCC can be classified as type 1, for which PBRM1 mutations are linked to poor survival but type 2 PRCC has increased expression of glycolysis and metabolism-related mRNA transcripts[2]. ccRCC tumors with VHL mutations show overexpression of vascular endothelial growth factor (VEGF) and hypoxia-inducible factors (HIFs) can contribute to angiogenesis and cancer progression. Similarly, some RCCs show hyperactivation of the serine/threonine kinase mammalian target of rapamycin (mTOR), which can lead to the overproduction of VEGF. VEGFR inhibitors and anti-VEGF antibodies have been tested as therapies for RCC, and the anti-VEGF antibody bevacizumab has been approved for use in combination with IFN-α for metastatic RCC[4]. The mTOR inhibitors everolimus and temsirolimus have also been approved for the treatment of RCC, typically in combination with tyrosine kinase inhibitors (TKIs)[5]. Combination therapies that target VEGF and mTOR are considered more effective since they work in concert to target tumor growth and vascularization, whereas sequential treatments are typically associated with a greater likelihood of tumors developing resistance[6]. Unfortunately, these combination therapies are associated with undesirable toxicities and have not been linked with durable responses[7]. Notably, an HIF-2α inhibitor, belzutifan, has shown good overall response rate and duration of response and has been approved by the FDA in 2021 for use in adults with VHL-associated RCC and in patients with advanced RCC who have been treated with anti-VEGF therapy and anti-PD-1/PD-L1 therapy[8]. Combination therapies with belzutifan and other targeted agents or ICB are currently being evaluated in clinical trials[9]. Advances in immunotherapy have led to transformative treatment options for RCC. Immune checkpoint blockade (ICB) has been one promising treatment strategy, and the FDA approved the use of anti-PD-1/PD-L1 (nivolumab) for the treatment of advanced ccRCC in 2015[10]. A follow-up study showed that anti-PD-1 was associated with the best clinical benefit in ccRCC carrying loss-of-function mutations in PBRM1, which appears to affect tumor expression profiles in such a way to maintain responsiveness to checkpoint blockade[11]. Cytotoxic T-lymphocyte-associated protein 4 (CTLA-4) is another checkpoint molecule targeted for checkpoint blockade with the monoclonal antibody ipilimumab. Similar to PD-L1 blockade, CTLA-4 blockade was associated with a partial response against metastatic RCC. Combination therapies have shown much more durable responses in patients with advanced RCC, and the FDA approved the use of nivolumab plus ipilimumab for the treatment of intermediate or high-risk metastatic RCC in 2018[12]. ICB in combination with TKI have also been approved for advanced disease [13,14,15,16]. Unfortunately, PD-1/PD-L1 blockade has been less successful for PRCC[17] and ChRCC[18], and further studies are needed to identify therapeutic targets in these forms of RCC. Clinical studies with other checkpoint blockade targets are currently underway and are likely to provide new treatment options to RCC patients[19]. The future of RCC therapies relies on the identification of new molecules or pathways in tumor cells that can be targeted therapeutically without causing toxicity or promoting resistance. Advances in single-cell omics are leading the way in terms of target identification and understanding how different forms of RCC progress.
Choosing the RIGHT Model - Syngeneic versus Humanized Mouse Models
Mouse models have been the workhorses of preclinical immuno-oncology (IO) research, and advances in mouse model development have expanded to applications for nearly all types of solid tumors and hematological malignancies. Preclinical evaluation of experimental immunotherapies has been advanced by syngeneic and humanized mouse models. Syngeneic mice are one of the most established types of models used in cancer research, whereas humanized mice are a contemporary mouse model that has been critical to the screening of immunotherapeutic agents. Here we highlight features of syngeneic and humanized mouse models and define which models are most relevant to different phases of preclinical IO research. Syngeneic Tumor Models Syngeneic tumor models are created by transplantation of tumor cell lines into immunocompetent mice with the same genetic background as the cell line.[1] Tumors can be transplanted intravenously or subcutaneously into mice and typically grow rapidly over several weeks. Different types of tumor cell lines can be used in this type of model, including spontaneous, transgenic, or carcinogen-induced tumor cell lines. Syngeneic mouse models are best suited for screening novel IO agents or gaining insight into anti-tumor responses in the context of an intact immune system. Given the rapid growth of tumors in syngeneic mice, these models are less well suited to studying early events in tumor growth associated with cancer stem cells or understanding the contributions of heterogeneous tumor microenvironments, and these models typically do not recapitulate the mutational heterogeneity observed in human tumors.[2] Champions Oncology offers an extensive suite of syngeneic models, meticulously curated to support your groundbreaking investigations within a robust and intact immune environment. We harness advanced biomedical techniques to deliver comprehensive data with accurate and reliable experimental endpoints: cutting-edge flow cytometry for detailed characterization of immune cell populations, western blot analysis to quantify the expression of pivotal cancer and immune signaling proteins, immunohistochemistry for the precise localization of these proteins within the tissue architecture. Explore our syngeneic tumor model offering here. Humanized Tumor Models Humanized tumor models are a more recent addition to preclinical IO research that provide valuable insight into how individual tumors from patients (xenografts) respond to experimental therapies. Prior to the development of humanized mouse models, human xenograft models were used for screening cytotoxic or immunotherapeutic agents like chimeric antigen receptor (CAR) T cells[3], and these models use human tumor cell lines or patient-derived specimens transplanted into immunocompromised host mice. Different immunocompromised models can be used, including athymic mice that lack T cells or severe combined immunodeficiency (SCID) models that lack all adaptive immune responses. Humanized mice have been engineered from immunocompromised mouse strains that include genetic mutations in other adaptive immune functions that allow for the engraftment of human hematopoietic cells. The NOD/SCID IL2rγ chain knockout (NSG) mouse (NOD.Cg-Prkdcscid Il2rgtm1Wjl/SzJ) is one of the most used combined immunodeficiency models that can be engrafted with human hematopoietic cells and primary human tumors.[4] These patient-derived xenograft (PDX) models are useful for evaluating experimental IO therapies in the context of the human immune system and can use human immune cells from the same or different donor as the tumor source. PDX models are suited to evaluating experimental therapies in the context of a genetically heterogeneous tumor and better recapitulate aspects of the tumor microenvironment. (Download our case study "Using Champions' Prostate PDX Models for Preclinical Validation of a Novel, PSMA-directed BiTE" to see how Amgen used our humanized models to test a novel PSMA-targeted BiTE) Tumors can be grafted either orthotopically or subcutaneously and this also impacts how tumors grow and respond to experimental treatments.[5] Given the heavily modified nature of the NSG immune system, these models do not always reflect responses observed in humans during clinical trials. Nonetheless, NSG mice and similarly modified humanized mice offer valuable insights into the efficacy of IO candidates. Champions Oncology's state-of-the-art humanized mouse models are tailored to harness the power of the human immune system. Our portfolio offers a suite of options crafted to empower your scientific investigation: engraftment of cell therapies, adoptive transfer of human PBMCs and NK cells, engraftment of CD34+ hematopoietic stem cells from cord blood for full reconstitution of the human immune system. Learn more about our humanized models offer here. Mouse models are constantly being refined and improved to better reflect human physiology. Both syngeneic and humanized mouse models serve as valuable tools for preclinical IO research and accelerate the screening and evaluation of novel therapeutics.
