Western blotting is a decades-old laboratory technique that is used to detect specific proteins from cell culture, tissue, or blood specimen. The term “western blot” is a twist on the Southern blotting method developed by Edwin Southern, which is used to detect DNA and shares methodological similarities with western blotting. The western blot method was first described by Harry Towbin in 1979 but the term “western blot” was coined by W. Neal Burnette in 1981[1],[2]. Since its initial description, the application of western blotting to all fields of biological and biomedical research has been broad because it is a straightforward and robust method for detecting specific proteins. Here we provide an overview of the western blot method and highlight the current application of western blotting in preclinical oncology research.
Western Blot Basics
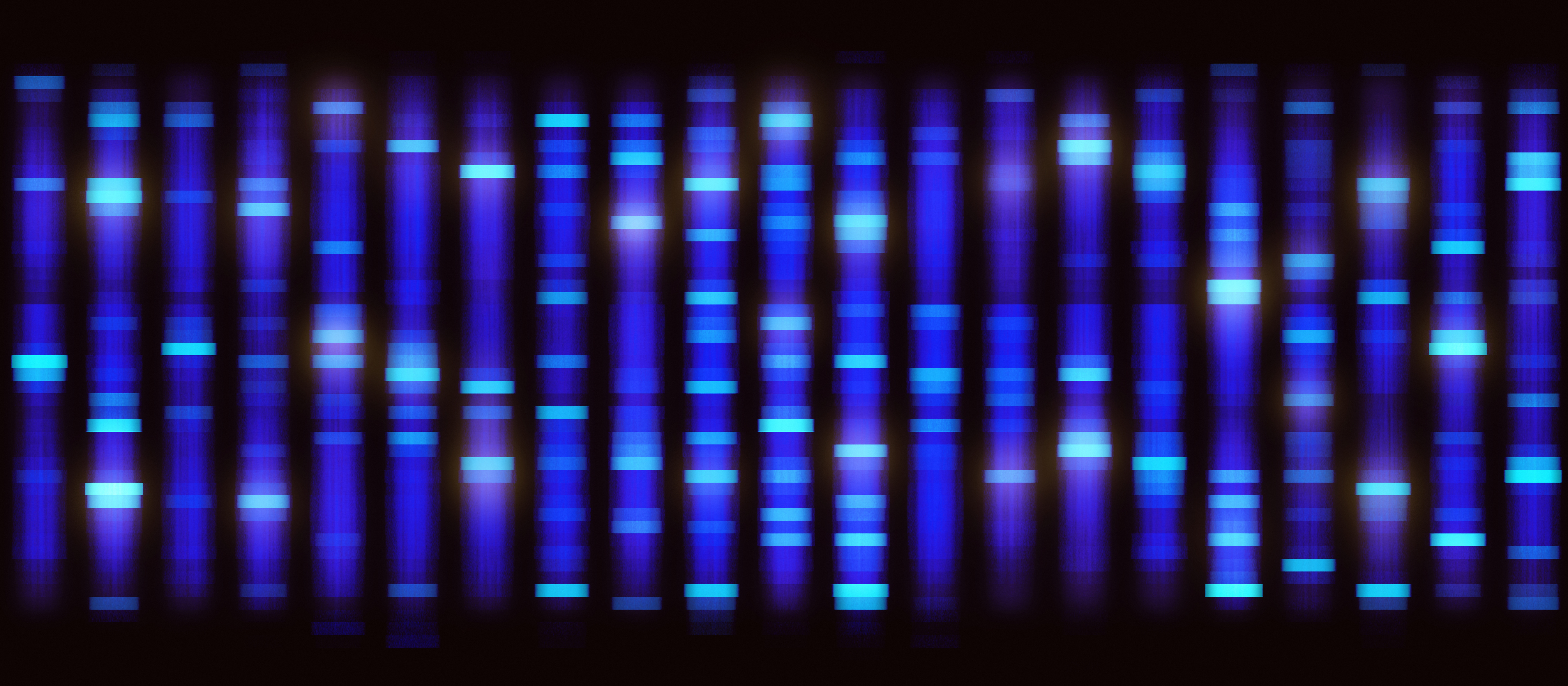
The western blot technique can be used to separate and identify a specific protein using three major steps:
1. Size separation using gel electrophoresis,
2. Transfer to a solid membrane, and
3. Detection with a specific antibody.
Most western blot methods begin with a lysate of cells or tissue, which releases a mix of proteins that are separated by molecular weight using gel electrophoresis. Sodium dodecyl sulfate polyacrylamide gel electrophoresis (SDS-PAGE) is the most common type of gel electrophoresis for western blotting and includes a protein denaturation step prior to gel electrophoresis such that proteins are separated by molecular weight. The SDS buffer causes proteins to become negatively charged so electrophoresis allows for migration of proteins from smallest to largest weight towards a positive charge. After gel electrophoresis, proteins are transferred to a solid membrane, usually polyvinylidene difluoride or nitrocellulose, using electroblotting or a slower alternative method based on capillary action. This membrane can now be probed with a primary antibody specific to a protein of interest and the primary antibody is visualized using a secondary antibody that recognizes a species-specific region of the primary antibody and is conjugated with a chemiluminescence substrate for visualization. Other less common visualization methods use colorimetric substrates or radioactive labels on secondary antibodies.

Application of Western Blotting to Preclinical Studies
Why is western blotting a method that is still used after more than forty years since its development? Western blotting remains a reliable, affordable, and practical method for detecting specific proteins. The application of western blotting in preclinical oncology research allows to validate high throughput single-cell RNA sequencing or proteomics methods that detect elevated proteins associated with specific cancers[3]. Western blotting can also validate tissue microarray and immunohistochemistry findings with respect to specific proteins that are overexpressed in tumor tissue. Together these data can be used toward developing prognostic biomarkers for cancers, such as measuring overexpression of Kin of IRRE-like Protein 1 (KIRREL) in breast cancer and precancerous tissue[4]. Western blotting is also a useful tool for understanding molecular mechanisms that drive cancer progression by measuring expression of critical proteins such as HMGB1, cyclins, and various oncogenes[5],[6].
Western blot has made a leap into the twenty-first century with advancements in process workflow and sensitivity using new platforms like ProteinSimple’s JESS System. Western blot will continue to be a workhorse for detecting and monitoring specific protein expression and the application of western blotting continues to be broad in preclinical and clinical oncology studies related to understanding oncogenesis and defining potential tumor biomarkers.