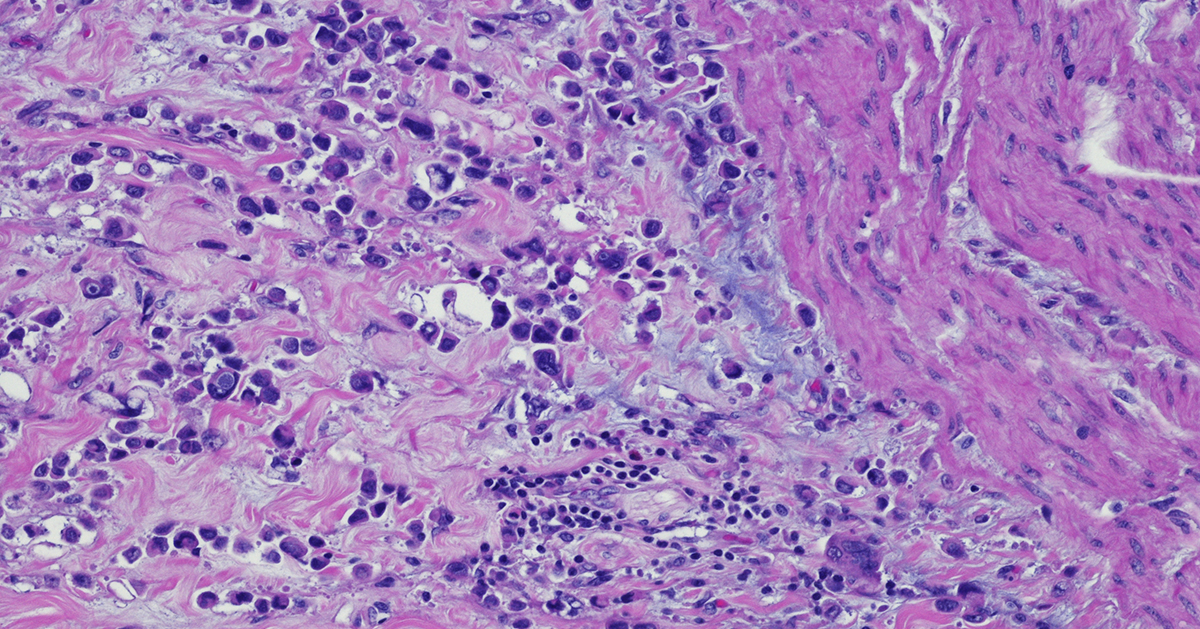
Immunohistochemistry (IHC) is based on antigen–antibody binding to directly visualize the status of markers of interest in tissues. IHC provides a huge amount of information and is now indispensable in many fields including pathology, cancer biology, and drug discovery.
IHC has been and continues to be analyzed by pathologists using microscopy. For pathologists in everyday practice or in the research context, IHC is an essential tool to interpret the pathophysiology of diseased tissues. Furthermore, IHC is also a crucial tool for biomarker discovery validation leading to personalized medicine. It provides information about the localization and the abundance of the target protein, both within tissue and at the sub-cellular level.
More recently, growing evidence of the importance of spatial information enclosed in the tissue for patient stratification leading to improved clinical outcomes further boosted the development and standardization of many different and improved image analysis methods.
This is particularly true in the field of immune-oncology and checkpoint inhibitors therapeutic development, where the presence of immune cells in the tumor correlated with positive clinical outcomes in many different types of cancer.
IHC Analysis and Scoring Methods
IHC results can be variable in both the percentage of positive detected cells as well as in protein expression intensity. The spatial distribution of the staining is also very variable. Sometimes the staining is widely spread out across the tissue while other times it is localized in small specific areas. The heterogeneity of the tissues reflected in the IHC staining needs to be classified, and more standardized scoring methods were needed to use this powerful tool in the clinic to stratify patients and improve outcomes.
In fact, to have a diagnostic and prognostic value, the biomarkers detected by IHC need to be quantified and expressed in numerical values. Different methods have been developed to quantify IHC biomarker staining for diagnostic and therapy design. So far, a universally accepted standardized unbiased method to quantify IHC does not exist. Instead, for each tumor type and correlated therapeutic regimen a specific method with a defined antibody and protocol has been developed.
The most common quantification methods are combinative semi-quantitative and percentage scoring methods.1
In the percentage scoring method, the relative immune-positive cell percentage in relation to the total number of cells is evaluated and reported using numbers from 0 to 9 where each unit increase represents 10% positive staining increments. For example, if the percentage of positive cells is between 0% and 9%, the score will be 0; if it is between 10% and 19%, the score will be 1; and so on. This method is preferable in large studies to avoid batch effects and can overcome interpretation errors in the evaluation of the positive cells. A limitation is that it does not include the staining intensity which in some cases could be important for patients’ stratification.2
Combinative semi-quantitative scoring is the most used method in the current prognostic biomarker research, providing a combined positive score incorporating both quantitative and qualitative assessments. In addition to the quantitative assessment of the relative immune-positive cell percentage described above, staining intensity is also assessed. Using the combinative semi-quantitative scoring method, the intensity is usually scored from 0 to 3 with 0 indicating negative staining, 1+ weakly positive staining, 2+ moderately positive, and 3+ strongly positive staining.2
Although IHC can be evaluated both quantitatively and qualitatively, an enormous number of variations is possible. This is due to the high number of diverse staining localization and spatial combinations. This leads to divergent interpretations among pathologists and discrepancies within pre-clinical and clinical studies evidencing the need for a more uniform system to interpret and quantify IHC data.
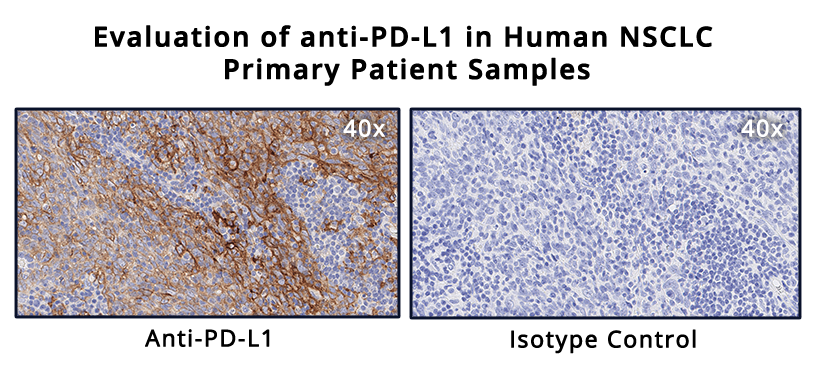
Testing for PD-L1 in metastatic NSCLC
PD-L1 expression is estimated in different manners depending on the type of cancer. Interestingly, in different indications, the presence of PD-L1 is not alone indicative of therapeutic efficacy. Different clinical trials demonstrated that, for different tumor types, PD-L1 localization correlated with treatment efficacy if PD-L1 is expressed in only immune cells, only cancer cells, or both. Thus, the IHC’s ability to localize the staining is fundamental.3
As an example of IHC use in the clinic, evaluation of PD-L1 expression helps identifying metastatic NSCLC patients who are eligible for treatment with pembrolizumab. In fact, in NSCLC, PD-L1 is a proven biomarker for patient response to pembrolizumab.3,4
In general, PD-L1 staining by IHC is evaluated using a combined positive score (CPS) and tumor proportion score (TPS) depending on the cancer type. The CPS score is calculated by counting the PD-L1 positive tumor cells and mononuclear inflammatory cells, then dividing this number by the total tumor cells, and multiplying by 100.4 CPS is used in the clinic to identify candidates for treatment with pembrolizumab in indications such as gastric cancer.3
In advanced NSCLC,4 the TPS scoring method is preferred to evaluate PD-L1 expression. TPS is calculated as the number of PD-L1 positive tumor cells divided by the total number of tumor cells and then multiplied by 100.
Interestingly, to determine the eligibility of a patient for pembrolizumab treatment, only PD-L1 membrane staining is scored while staining intensity is not included in the score, and only the tumor cells and not the immune cells are evaluated. In this case, a competent, trained pathologist is critical to evaluate tumor heterogeneity and discriminate between tumor cells and infiltrating macrophages that are usually positive for PD-L1. For this PD-L1 staining scoring method to be reliable, at least 100 viable tumor cells must be evaluated within the patient’s tissue.2, 4
IHC has proven to be a powerful tool for oncology clinical practice. However, in order to fully leverage the information enclosed within the tissue in a clinical setting, and given the complexity of the IHC staining interpretation, crucial are both the pathologist’s training and expertise in the specific disease evaluation.
[1] Dabbs DJ. 2014. Diagnostic immunohistochemistry: theranostic and genomic applications. 4th ed. Philadelphia: Elsevier Saunders.
[2] Kim S, Roh J, Park C. 2016. Immunohistochemistry for Pathologists: Protocols, Pitfalls, and Tips, J Pathol Transl Med 50(6):411-418.
[4] Inamura K. 2018. Update on Immunohistochemistry for the Diagnosis of Lung Cancer, Cancers10(3), 72;
[3] Davis AA, Patel VG. 2019. The role of PD-L1 expression as a predictive biomarker: an analysis of all US Food and Drug Administration (FDA) approvals of immune checkpoint inhibitors, j. immunotherapy cancer 7, 278
.