Bladder cancer is a relatively common form of cancer that is defined as either pre-invasive or invasive, and non-muscle invasive bladder cancer (NMIBC) is the most commonly diagnosed subtype[1]. NMIBC is typically treated by surgical resection and/or intravesical delivery of chemo- or immunotherapy-based adjuvant treatment, and long-term efficacy is monitored by urine testing or cystoscopy. Muscle-invasive bladder cancer (MIBC) is relatively resistant to current treatment options and occurs more frequently in men. MIBC also has high rates of morbidity and mortality, and novel therapies or combination therapies are being developed to better treat this form of bladder cancer.
Here we highlight recent findings about invasive bladder cancer biology and how these observations are informing the development of new therapies.
Mutation Landscape
Analysis of MIBC genomes has revealed a high mutation rate in tumors that approaches rates seen in melanoma and lung cancer[2]. The mutational signature of many MIBC tumors is consistent with mutations associated with APOBEC (apolipoprotein B mRNA-editing enzyme, catalytic polypeptide-like) cytidine deaminase, and many of the mutations are clonal, which suggests that the effects of APOBEC expression in bladder tissue occurs early in tumorigenesis. Non-invasive and invasive bladder tumors are also often classified as being highly heterogeneous, and tumor histology can be extremely variable and includes squamous, plasmacytoid, micropapillary, and small-cell carcinoma. This molecular and histological heterogeneity presents a challenge in subtyping tumors based on gene expression profiles and provides insight into why it can be difficult to identify targeted therapies that are effective against bladder cancers.
Several genes are commonly mutated in both NMIBC and MIBC, but mutation rates for some of these genes, including MLL2 and TP53, are higher in invasive tumors[3]. TP53 mutations appear to be a critical factor associated with the transition to its invasive form. Mutations have also been identified in genes associated with the sister chromatid cohesion and segregation process (STAG2, ESPL1, FGFR3, and TACC3)[3] and chromatin remodeling (UTX, MLL-MLL3, CREBBP-EP300, NCOR1, ARID1A, and CHD6)[4].
Immunotherapy Insights
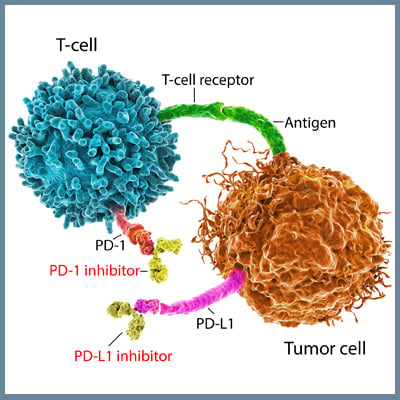
NIMBC is one of the first forms of cancer treated with an immunotherapy approach in which the attenuated tuberculosis bacterial vaccine, Bacillus Calmette-Guérin (BCG), is delivered intravesically to the bladder and triggers an immune response that inhibits tumor growth[5]. Recently, immune checkpoint inhibitor-based treatments have been examined as therapeutic options for different bladder cancers. Expression of the PD-L1 immune checkpoint molecule has been detected on both NIMBC and MIBC tumor cells and tumor-infiltrating mononuclear cells. Antibody-based blockade of PD-1/PD-L1 interactions restores anti-tumor responses mediated by T cells. Atezolizumab was the first PD-L1 inhibitor approved for the treatment of locally advanced/ metastatic urothelial carcinoma (LA/mUC) that has progressed or after cisplatin-based chemotherapy[6], and five different PD-L1/PD-1 inhibitors have been approved for this form of bladder cancer. Other checkpoint inhibitors that target different molecules have not shown significant improvements in overall survival or progression-free survival. Clinical trials currently underway are examining the efficacy of combinations of checkpoint inhibitors or the use of these inhibitors as an adjuvant therapy to surgical resection or chemotherapy. In addition, therapies that target specific mutations, such as FGFR, HER2, or chromatin-modifying genes, are also under investigation, and the FGFR inhibitor erdafitinib was recently approved for the treatment of LA/mUC[7][8].
Bladder cancer treatment outcomes continue to advance because of insights into the basic biology of bladder cancer, and a better understanding of molecular and immunological mechanisms associated with anti-tumor responses. The many preclinical and clinical studies currently underway for bladder cancer will contribute valuable information to new therapeutic options, especially for patients with refractory or invasive forms of bladder cancer.
[1] Knowles MA, Hurst CD. Molecular Biology of Bladder Cancer: New Insights into Pathogenesis and Clinical Diversity. Nat Rev Cancer. 2015;15:25–41.
[2] Robertson AG, Kim J, Al-Ahmadie H, et al. Comprehensive Molecular Characterization of Muscle-Invasive Bladder Cancer. Cell. 2017;171(3):540-556.e25.
[3] Guo G, Sun X, Chen C, et al. Whole-Genome and Whole-Exome Sequencing of Bladder Cancer Identifies Frequent Alterations in Genes Involved in Sister Chromatid Cohesion and Segregation. Nat Genet. 2013;45(12):1459-1463.
[4] Gui Y, Guo G, Huang Y, et al. Frequent Mutations of Chromatin Remodeling Genes in Transitional Cell Carcinoma of the Bladder. Nat Genet. 2011;43(9):875-878.
[5] Herr HW and Morales A. History of Bacillus Calmette-Guerin and Bladder Cancer: An Immunotherapy Success Story. J Urol. 2008;179.1: 53-56.
[6] Bellmunt J, Powles T, Vogelzang NJ. A Review on the Evolution of PD-1/PD-L1 Immunotherapy for Bladder Cancer: The Future is Now. Cancer Treat. Rev. 2017;54:58-67.
[7] Osterman CK, Milowsky MI. New and Emerging Therapies in the Management of Bladder Cancer. F1000Res. 2020;9:F1000 Faculty Rev-1146. 1
[8] Loriot Y, Necchi A, Park SH, et al. Erdafitinib in Locally Advanced or Metastatic Urothelial Carcinoma. N Engl J Med. 2019 Jul 25;381(4):338-348.