Recent advances in immunotherapy have led to the development of targeted therapies for treating lymphoma and leukemia. One such therapy is CD19-targeted therapy, which is widely used as a frontline treatment for B cell lymphoma and leukemia. However, despite many patients initially respond well to this therapy relapse remains the major obstacle to be addressed. In this blog post, we will explore the various mechanisms underlying relapse to CD19-targeted therapies in patients with B cell lymphoma and leukemia.
Antigen-positive Relapse:
Early relapse is usually associated with short CAR-T cell persistence. This can be due to poor T cell quality, but, more importantly, it is determined by the costimulatory domain in the CAR. In fact, domains such as 4-1BB have been proven to significantly prolong the persistence of CAR-T cells[1]. In addition, the gene editing technology used to engineer the T cells can make a difference in CAR-T cell persistence. Combination therapies with checkpoint inhibitors have also been shown to prolong response duration and remission[1,2].
Immune Evasion Mechanisms:
One of the primary mechanisms of acquired resistance to CD19-targeted therapies is immune evasion. Cancer cells can develop various mechanisms to evade immune surveillance and avoid being targeted by the therapy. For example, cancer cells may downregulate the expression of CD19 on their surface, thereby reducing the effectiveness of the therapy. Alternatively, they may upregulate other immune checkpoint molecules or express inhibitory proteins that prevent immune cells from recognizing and eliminating them[3].
Antigen Loss Mechanisms:
Another mechanism of acquired resistance is antigen loss. CD19-targeted therapies are designed to bind to and eliminate cancer cells that express the CD19 antigen. However, cancer cells can develop mutations that lead to loss of CD19 expression, rendering the therapy ineffective. This mechanism of resistance has been observed in both lymphoma and leukemia patients, and it is a significant challenge in the development of novel therapies[4].
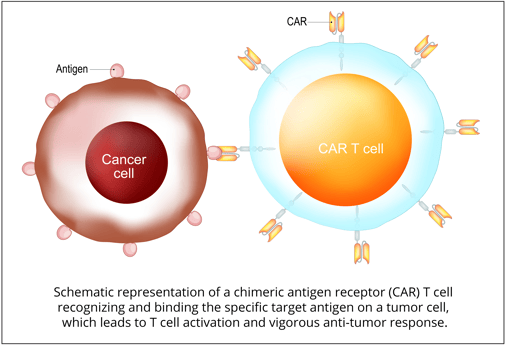
Development of Alternative Pathways:
Cancer cells that survive CD19-targeted therapies can develop alternative pathways for survival and growth. These pathways can bypass the effects of the therapy and allow cancer cells to continue to proliferate. Examples of such mechanisms include the activation of alternative signaling pathways and the upregulation of survival factors. Understanding these adaptive mechanisms is crucial in devising new therapeutic strategies to overcome acquired resistance[1,3].
Tumor Microenvironment:
The tumor microenvironment (TME) plays a critical role in acquired resistance to CD19-targeted therapies. The TME is a complex milieu of immune and stromal cells that interact with cancer cells to promote tumor growth and survival. The TME can also influence the response of cancer cells to therapy. For example, the presence of immunosuppressive cells in the TME can reduce the effectiveness of CD19-targeted therapies[1,5].
Genetic Factors:
Genetic factors also play a role in acquired resistance to CD19-targeted therapies. For example, mutations in genes involved in DNA repair pathways can increase genomic instability and promote drug resistance. Identifying genetic factors that contribute to resistance can help to personalize therapy and improve outcomes[6].
Disease relapse and development of acquired resistance to CD19-targeted therapies in patients with B cell lymphoma and leukemia pose a significant clinical challenge. To overcome this challenge, we need to find ways to optimize CAR-T cell design to produce more effective therapies and understand the various mechanisms of resistance to be able to develop new therapeutic strategies that target these mechanisms. Research efforts are underway to identify novel therapeutic targets and personalized treatment approaches that can overcome acquired resistance and improve outcomes for patients[7].
Continuous improvements in our understanding of acquired resistance and relapse to CD19-targeted therapies will pave the way for the development of more effective and durable treatments for these diseases. To overcome this challenge, access to relevant preclinical models is key to develop alternative or synergistic therapeutic approaches. Champions Oncology offers a large cohort of B-cell lymphoma and leukemia models, including primary samples from patients who progressed after CD19-targeted therapies, to accelerate the development of novel therapeutic approaches to improve B-cell lymphoma and leukemia patients' lives.
- Shah NN, et al. Mechanisms of resistance to CAR T cell therapy. Nat Rev Clin Oncol. 2019 Jun;16(6):372-385. doi: 10.1038/s41571-019-0184-6. PMID: 30837712; PMCID: PMC8214555.
- Sterner RC, et al. CAR-T cell therapy: current limitations and potential strategies. Blood Cancer J. 2021 Apr 6;11(4):69. doi: 10.1038/s41408-021-00459-7. PMID: 33824268; PMCID: PMC8024391.
- Plaks V, et al. CD19 target evasion as a mechanism of relapse in large B-cell lymphoma treated with axicabtagene ciloleucel. Blood. 2021 Sep 23;138(12):1081-1085. doi: 10.1182/blood.2021010930. PMID: 34041526; PMCID: PMC8462361.
- Sworder BJ, et al. Determinants of resistance to engineered T cell therapies targeting CD19 in large B cell lymphomas. Cancer Cell. 2023 Jan 9;41(1):210-225.e5. doi: 10.1016/j.ccell.2022.12.005. Epub 2022 Dec 29. PMID: 36584673; PMCID: PMC10010070.
- Frey NV, et al. Optimizing Chimeric Antigen Receptor T-Cell Therapy for Adults With Acute Lymphoblastic Leukemia. J Clin Oncol. 2020 Feb 10;38(5):415-422. doi: 10.1200/JCO.19.01892. Epub 2019 Dec 9. PMID: 31815579; PMCID: PMC8312030.
- Chen GM, et al. Characterization of Leukemic Resistance to CD19-Targeted CAR T-cell Therapy through Deep Genomic Sequencing. Cancer Immunol Res. 2023 Jan 3;11(1):13-19. doi: 10.1158/2326-6066.CIR-22-0095. PMID: 36255409; PMCID: PMC9808313.
- Atilla PA, et al. Resistance against anti-CD19 and anti-BCMA CAR T cells: Recent advances and coping strategies. Transl Oncol. 2022 Aug;22:101459. doi: 10.1016/j.tranon.2022.101459. Epub 2022 May 23. PMID: 35617812; PMCID: PMC9136177.