Colorectal cancer (CRC) is a leading cause of cancer deaths globally. Advances in early detection have improved survival rates, but patients diagnosed with metastatic CRC still have stubbornly poor 5-year survival rates.
Standard treatments for CRC include surgery, chemotherapy, and radiotherapy, but alternative immuno-oncology therapies are showing promising results in CRC patients. Here we highlight advances in immuno-oncology therapies that are being used to treat CRC patients or are being pursued in preclinical and clinical studies.
CRC Subtypes
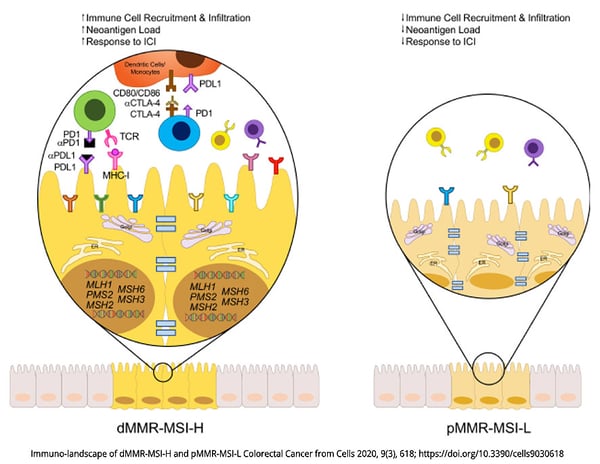
Molecular genetic analysis of CRC tumors is a critical diagnostic tool for classifying and treating this cancer. CRC tumors are typically classified as mismatch repair-deficient/microsatellite instability-high (dMMR—MSI-H) tumors, which have a high overall mutation burden, or mismatch repair-proficient/microsatellite instability-low (pMMR—MSI-L) tumors, which have a lower mutation burden[1]. Defects in MMR are associated with the accumulation of mutations and are caused by defects in mismatch repair proteins, and these defects are typically detected by frame-shift mutations in DNA repeat regions known as microsatellites. Mutations in the BRAF oncogene, particularly the activating V600E mutation, comprise a distinct subset of CRC. BRAF is a component of the mitogen-activated protein kinase (MAPK) pathway that normally functions downstream of the epidermal growth factor receptor to regulate transcription of genes involved in cellular growth and survival, but the BRAF-V600E mutation results in constitutive activation of BRAF and uncontrolled cellular proliferation and tumor growth[2].
Immuno-Oncology Interventions
Immuno-oncology approaches that target immune checkpoint blockade have proven effective for several cancers, including CRC. T cells can initiate effective anti-tumor responses under ideal conditions, but the immunosuppressive tumor microenvironment of some cancer types inhibits T cell activation, usually through engagement of immune checkpoint molecules like PD-1 and CTLA4. For more than a decade, the development and use of immune checkpoint inhibitors (ICIs) has transformed the treatment of melanoma[3,4] and non-small cell lung cancer[5,6]. The first FDA-approved treatments include a monoclonal antibody (mAb) that targets CTLA4 (ipilimumab) and two mAbs that target PD-1 (pembrolizumab and nivolumab). These early studies suggested that tumors with high mutation burdens respond well to ICI, which may be due in part to the generation and presentation of tumor neoantigens that are recognized as non-self and can be targeted by cytotoxic T cells[7].
CRC tumors with the dMMR—MSI-H signature have a high mutational burden and typically have a high level of CD4+ and CD8+ tumor-infiltrating lymphocytes (TILs). These cells have shown elevated expression of PD-1, PD-L1, and CTLA4, which suggests that they may respond well to ICIs[8]. Indeed, several recent clinical studies have shown improvements in overall survival and progression-free survival in patients treated with individual or combined ICIs that target PD-1. Of note, interim results from a phase II trial that enrolls patients with MMRd locally advanced rectal cancer to receive neoadjuvant dostarlimab, a PD-1 inhibitor, showed sustained complete response 1 year after the end of treatment in all patients[9]. Other current studies are evaluating next-generation PD-1 inhibitors combined with chemotherapy and/or other biologics such as an anti-VEGF and anti-EGFR mAb[10].
In contrast, patients with pMMR–MSI-L have shown poor responses to PD-1 or CTLA4 blockade alone or in combination, which has led to the development of trials that explore different treatment combinations, including inhibitors of the MAPK pathway or angiogenesis[11]. Promising results have been presented in a phase I/II clinical trial (NCT04017650) in which patients with MSI-L and BRAFV600E were treated with BRAF+EGFR inhibitors, to induce a transient MSI-H phenotype, plus anti-PD-1 antibody[12].
Next-Generation Treatments
Several cutting-edge immuno-oncology therapies are being explored for the treatment of CRC. Beyond combinations of individual antibodies, researchers are engineering bispecific antibodies that bind to tumor cells and T cells simultaneously to enhance anti-tumor T cell responses. One such bispecific antibody, CEA-TCB, is being tested in phase I trials alone or in combination with anti-PD-L1 to treat metastatic CRC[13]. Another novel approach includes adoptive cell therapies like chimeric antigen receptor (CAR) T cells, which are T cells collected from the tumor tissue or peripheral blood of a patient and engineered to bind to tumor antigens and potentiate anti-tumor responses. Oncolytic virus and bacteria-based vaccines are also being studied as potential CRC treatments.
Besides treatments designed on the tumor's intrinsic genetic background, much effort is being put into deciphering the tumor microenvironment, with a particular focus on its interplay with the gut microbiota to modulate inflammation and immune response, known to be involved in the metastatic onset. These studies are leading to the identification of inflammatory/immune signatures that inform the therapeutic agents to be used to target the pro-oncogenic microenvironment and reactivate the immune system[14].
The advancement of immuno-oncology is already transforming the treatment of CRC and will contribute to better outcomes for all types of CRC in the decades to come.